
8. Multimerization of a Proline-Rich Antimicrobial Peptide, Chex-Arg20, Alters Its Mechanism of Interaction with the Escherichia coli Membrane
Highlights
•Multimers of the proline-rich antimicrobial peptide, ChexArg20, were prepared
•Increase in peptide valency alters its Escherichia coli membrane interaction
•Change is from membrane non-lytic to membrane disruption
•There is also simultaneous change from membrane hyperpolarization to depolarization
7. Polyvinyl Alcohol Nanofiber Formulation of The Designer Antimicrobial Peptide APO Sterilizes Acinetobacter Baumannii-Infected Skin Wounds in Mice
Native and designer cationic antimicrobial peptides are increasingly acknowledged as host defense molecules rather than true antimicrobials. Due to their ability to activate the innate immune system, these structures are used to treat uninfected and bacterially-infected wounds, including those harboring Acinetobacter baumannii.

6. C-Terminal Modifications Broaden Activity of the Proline-Rich Antimicrobial Peptide, Chex1-Arg20
A series of N- and C-terminal modifications of the monomeric proline-rich antimicrobial peptide, Chex1-Arg20, was obtained via different chemical strategies using Fmoc/tBu solid-phase peptide synthesis in order to study their effects on a panel of Gram-negative bacteria. In particular, C-terminal modifications with hydrazide or alcohol functions extended their antibacterial activity from E. coli and K. pneumoniae to other Gram-negative species, A. baumannii and P. aeruginosa. Furthermore, these analogues did not show cytotoxicity towards mammalian cells. Hence, such modifications may aid in the development of more potent proline-rich antimicrobial peptides with a greater spectrum of activity against Gram-negative bacteria than the parent peptide.

5. Proline-Rich Antimicrobial Peptides: Potential Therapeutics Against Antibiotic-Resistant Bacteria
The increasing resistance of pathogens to antibiotics causes a huge clinical burden that places great demands on academic researchers and the pharmaceutical industry for resolution. Antimicrobial peptides, part of native host defense, have emerged as novel potential antibiotic alternatives.
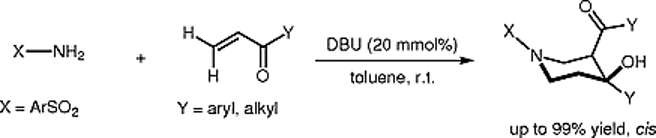
4. Synthesis of 3-Aroyl-4-hydroxy-4-arylpiperidine Derivatives by DBU-Catalyzed Reactions of Amines with Vinyl Ketones
In one-pot cascade reaction, a series of functionalized 3-aroyl-4-hydroxy-4-arylpiperidine derivatives were prepared from sulfonamides and vinyl ketones with good to excellent yields and diastereoselectivities. The reaction was catalyzed by the commercially available Lewis base 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and could be easily manipulated under mild condition.

3. An Asymmetric Organocatalytic Approach to Michael Reactions of Thiazolones and Nitroalkenes: Preparation of Compounds with Anti-Cancer Potency
We present a highly efficient strategy for obtaining a series of chiral 2,4-disubstituted thiazolone derivatives with excellent diastereo- and enantioselectivities through the creation of carbon- and nitrogen-substituted quaternary carbon stereocenters. With the chiral tertiary amine-thiourea catalyst developed by our group, the reactions could be performed smoothly at 1 mol-% catalyst loadings without any additive. Preliminary biological evaluation demonstrated that these analogues could inhibit cell proliferation in vitro significantly.
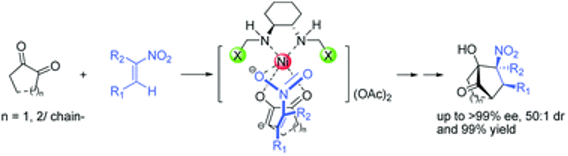
2. New Approach to The Preparation of Bicyclo Octane Derivatives via The Enantioselective Cascade Reaction Catalyzed by Chiral Diamine-Ni(OAc)2 Complex
A highly efficient catalyst system assembled from enantiomerically pure diaminocyclohexane and Ni(OAc)2 is, for the first time, used to catalyze the cascade Michael–Henry reaction of various diones and substituted nitroalkenes.

1. RETURN TO ISSUEPREVNOTENEXTWater-Compatible Iminium Activation: Highly Enantioselective Organocatalytic Michael Addition of Malonates to α,β-Unsaturated Enones
The highly enantioselective Michael addition of malonates to α,β-unsaturated ketones in water was reported to be catalyzed by a primary−secondary diamine catalyst containing a long alkyl chain. This asymmetric Michael addition process was found to be effective for a variety of α,β-unsaturated ketones.