2. New Approach to The Preparation of Bicyclo Octane Derivatives via The Enantioselective Cascade Reaction Catalyzed by Chiral Diamine-Ni(OAc)2 Complex
Abstract:
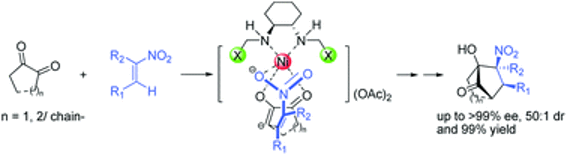
A highly efficient catalyst system assembled from enantiomerically pure diaminocyclohexane and Ni(OAc)2 is, for the first time, used to catalyze the cascade Michael–Henry reaction of various diones and substituted nitroalkenes. A series of polyfunctionalized bicyclo[3.2.1]octane derivatives containing four stereogenic centers are prepared with excellent enantioselectivities (up to >99% ee) and diastereoselectivities (up to 50 : 1 dr) with high yields. In addition, via this chiral diamine-Ni(OAc)2 catalyst system, the base-induced epimerization leading to the decrease of stereoselectivity can be prevented.
Li, W.; Liu, X.; Mao, Z.; Chen, Q.; Wang, R.* (2012): New approach to the preparation of bicyclo octane derivatives via the enantioselective cascade reaction catalyzed by chiral diamine-Ni(OAc)2 complex. Org. Biomol. Chem. 10, 4767-4773, DOI: 10.1039/C2OB25135C.